"Watch it wiggle, see it jiggle." Those of us old enough surely remember this TV jingle for Jell-O. And if you don't, chances are good that you've eaten the stuff at some point in your life. There are endless recipes that use Jell-O to create everything from simple squares to ornate molds that incorporate varied flavors, fruits and whipped toppings.
But here's the million-dollar question. What is Jell-O?
Advertisement
Jell-O is a brand-name of gelatin made by Kraft Heinz. It includes a few basic ingredients:
- water
- sugar or artificial sweetener
- fruit juices for flavoring (like strawberry concentrate)
- gelatin
- coloring
But what is it about Jell-O that lets you mold it into so many different shapes? In a word gelatin.
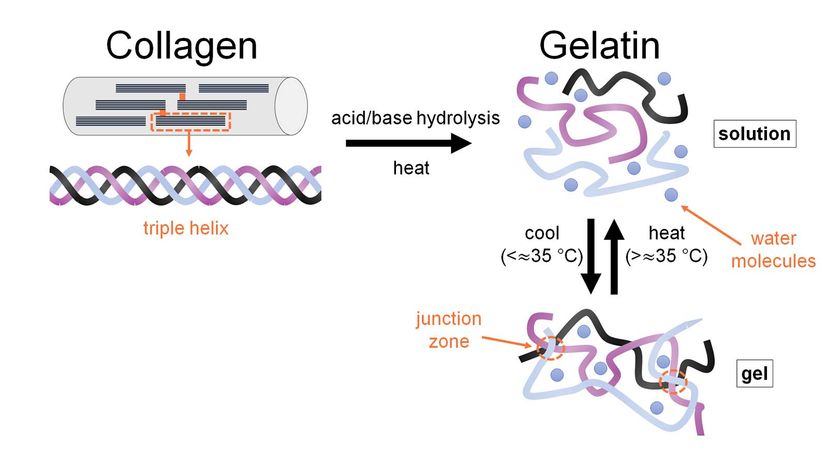
But what is gelatin? Gelatin is a processed version of a structural protein called collagen. It comes from animals, including humans, and makes up almost one-third of all the protein in the human body.
Collagen is a fibrous protein that strengthens the body's connective tissues and allows them to be elastic — that is, to stretch without breaking. As you get older, your body makes less collagen, and individual collagen fibers become increasingly cross-linked with each other. You might experience this as stiff joints from less flexible tendons, or wrinkles due to loss of skin elasticity.
Gelatin can come from the collagen in cow or pig bones, hides and connective tissues. The gelatin in Jell-O is most likely from pigskin.
Collagen doesn't dissolve in water in its natural form, so it must be modified to make gelatin. Manufacturers grind the pigskin and treat it with a strong acid for about 24 hours. Then the pretreated skin is boiled to unravel the protein bonds in the collagen. The resulting product is a gelatin solution.
There are, of course, safety controls at every step of the process ensure purity. That final solution is chilled into a jelly-like material, cut and dried. At this point, the dried gelatin — about 10 percent water — is ground. If it's going to make Jell-O, it will be ground into a fine powder.
How does this powder become the Jell-O we eat?
Advertisement