Cold marble countertops, swiveling leather stools and pink neon signs. A boy and a girl smile shyly as they share a fizzy-sweet treat through two long straws. Behind the counter, a teenage soda "jerk" in his trademark black bowtie and crisp white paper cap pulls bubbling carbonated water into tall chilled glasses, then blends in flavored syrups to create drug store favorites like phosphates and egg creams (no egg, no cream, lots of chocolate).
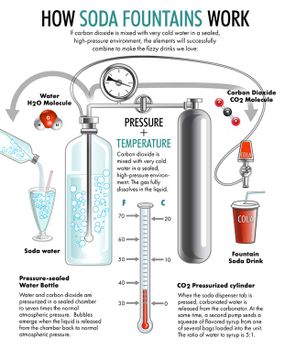
This is the classic American soda fountain experience of the 1940s. At the center of it is the fountain itself. Back in the late 19th century, the grandest soda fountains boasted long rows of silver gooseneck spigots dispensing pure carbonated water that could be hand-mixed with more than 100 flavored syrups, many derived from natural fruits, herbs and pungent roots: birch, lemon, ginger, cherry, pomegranate, gooseberry, nutmeg, grape, vanilla, peppermint and rose.
Advertisement
Today, the closest things to a soda fountain that most of us recognize are the familiar self-serve beverage machines in every fast-food restaurant and cafeteria. Instead of dispensing carbonated water and syrup separately to be mixed by hand, modern soda fountains are programmed to dispense a precise amount of fizzy water with sugary flavoring to achieve the patented taste of Coke, Sprite, Dr. Pepper, Mountain Dew and dozens more multinational brands.
With health concerns over the high sugar content of most commercial soft drinks, many people are opting to make their own sodas at home. The DIY soda boom is fueled by the popularity of the soda makers, affordable countertop appliances that can turn ordinary tap water into bubbling seltzer in seconds. Adventurous consumers can brew up their own pop concoctions using flavors provided by the manufacturers or whatever concoctions they like.
The public appetite for fizzy drinks started with spa-goers drinking water from naturally bubbling springs, which were thought to have curative powers. But the mass production of soda only started after people figured out how to make carbonated water in a machine. And that was harder to do than you'd think.
Advertisement